
"Understanding the interactions between nanomaterials and biological systems plays a crucial role in improving the effectiveness of nanomedicines and deepening understanding of the biological field. Fluorescence microscopy is a powerful optical imaging technique that can directly observe the behavior of fluorescently labeled nanomaterials in the microenvironment within cells. However, conventional fluorescence microscopy, such as confocal microscopy, has limited optical resolution due to the diffraction of light and therefore cannot provide precise details of nanomaterials with diameters less than 250 nm. Fortunately, the development of super-resolution fluorescence microscopy has overcome the limitations of resolution and enabled more comprehensive studies of nanocellular interactions. Here, we summarize recent advances in nanocellular interactions studied by various super-resolution microscopy techniques. ”
In order to fully understand the interaction of nanomaterials with intracellular domains, the role of physicochemical properties in determining the cellular binding, internalization, processing, and intracellular fate of nanomaterials must be understood in more detail, which requires multicolor imaging techniques with higher quality. Therefore, new methods that can overcome the optical resolution limitations of fluorescence microscopy are needed. Over the past few decades, super-resolution fluorescence microscopy or diffraction-limit fluorescence microscopy has been developed to allow images to be captured at higher resolutions beyond the diffraction limit with resolutions of around 5 nm. In particular, on October 8, 2014, the Nobel Prize in Chemistry was awarded to Eric Betzig, William. E. Moerner and Stefan W. Hell's "Development of Super-Resolution Fluorescence Microscopy," which brings "light microscopy to the nano-dimension," fills the gap between electron microscopy and fluorescence microscopy.
《Advances in super-resolution fluorescence microscopy for the study of nano–cell interactions》
01 Research results
1. Super-resolution microscope classification
Super-resolution microscopy can be broadly divided into two broad categories: "true" super-resolution techniques capture the information contained in the evicted wave and give a direct super-resolution image; and "functional" super-resolution techniques, which use smart experimental techniques and known limitations on the sample being imaged to reconstruct and produce deterministic (utilizing the response of a nonlinear fluorophore) or randomized (utilizing the complex temporal behavior of the fluorophore) super-resolution images. Super-resolution methods can also be identified by three well-known families, as shown in Figure 1, including (i) "structured illumination" SIM (ii) "stimulated emission loss" method STED; and (iii) a "single-molecule localization" method in which individual fluorescent molecules are positioned sequentially and the image is reconstructed by acquiring bright spots. Among them, SIM, STED, STORM, PALM, PAINT and other super-resolution technologies have been successfully applied to the biological field.
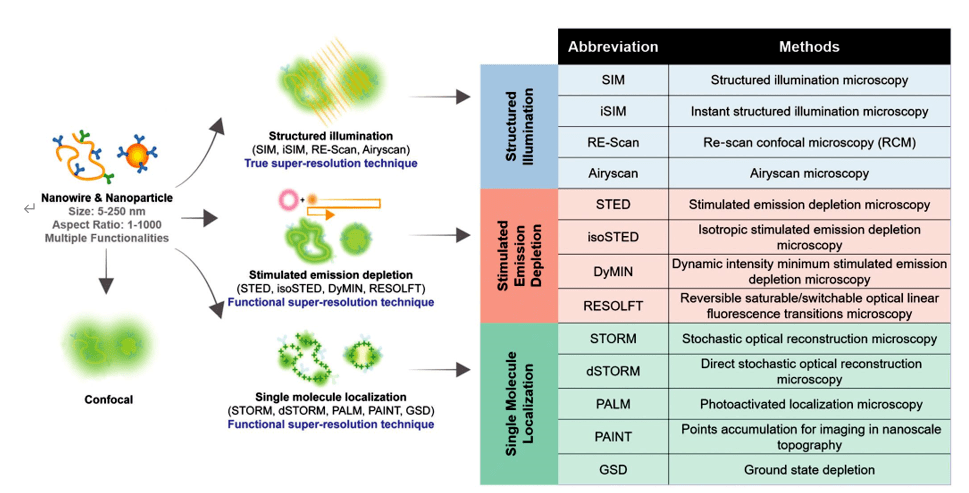
Figure 1. A catalog of super-resolution methods and descriptions of the representative mechanisms they use to observe nanomaterials, including structured illumination, stimulated emission loss, and single-molecule localization
2. Use super-resolution fluorescence microscopy to study nanobiological interactions
2.1 Structured Illumination Microscopy (SIM)
SIM is a super-resolution fluorescence microscopy method that improves spatial resolution by utilizing moiré fringes that contain otherwise unobservable structural information encoded in the viewing region. Specific gratings are used to form diffraction patterns at different angles, resulting in a Mohr pattern with additional information (Figure 1). Therefore, when using these multiple raster angles (more than three different angles), approximately twice as much information can be extracted from the sample. This information can be used to mathematically reconstruct a super-resolution image with a lateral resolution of 110 nm and an axial resolution of 250 nm. Methods such as instant SIM and nonlinear SIM have been able to achieve spatial resolution of 50 nm. SIM is widely used in nanobiological interaction studies due to its fast image acquisition at frame rates of more than 10 Hz per color channel. SIM-enhanced resolution increases the colocalization signal of nanomaterials with intracellular organelles observed in the image.
Detection of metal oxide nanoparticles in HeLa cells using confocal microscopy and SIM. They showed that when using SIM imaging, some regions in the images obtained by confocal microscopy that appeared to be colocalization of nanoparticles and lysosomes proved not to be true colocalization. Nanoparticles may be contained in other membrane-bound structures (e.g., endosomes) (Figure 2I). In this case, SIM is able to more accurately identify nanoparticles colocalized with lysosomes, which facilitates nanodrug carrier design that incorporates pH-triggered drug release for precise therapeutic payload delivery.
2.1 Structured Illumination Microscopy (SIM)
SIM is a super-resolution fluorescence microscopy method that improves spatial resolution by utilizing moiré fringes that contain otherwise unobservable structural information encoded in the viewing region. Specific gratings are used to form diffraction patterns at different angles, resulting in a Mohr pattern with additional information (Figure 1). Therefore, when using these multiple raster angles (more than three different angles), approximately twice as much information can be extracted from the sample. This information can be used to mathematically reconstruct a super-resolution image with a lateral resolution of 110 nm and an axial resolution of 250 nm. Methods such as instant SIM and nonlinear SIM have been able to achieve spatial resolution of 50 nm. SIM is widely used in nanobiological interaction studies due to its fast image acquisition at frame rates of more than 10 Hz per color channel. SIM-enhanced resolution increases the colocalization signal of nanomaterials with intracellular organelles observed in the image.
Detection of metal oxide nanoparticles in HeLa cells using confocal microscopy and SIM. They showed that when using SIM imaging, some regions in the images obtained by confocal microscopy that appeared to be colocalization of nanoparticles and lysosomes proved not to be true colocalization. Nanoparticles may be contained in other membrane-bound structures (e.g., endosomes) (Figure 2I). In this case, SIM is able to more accurately identify nanoparticles colocalized with lysosomes, which facilitates nanodrug carrier design that incorporates pH-triggered drug release for precise therapeutic payload delivery.
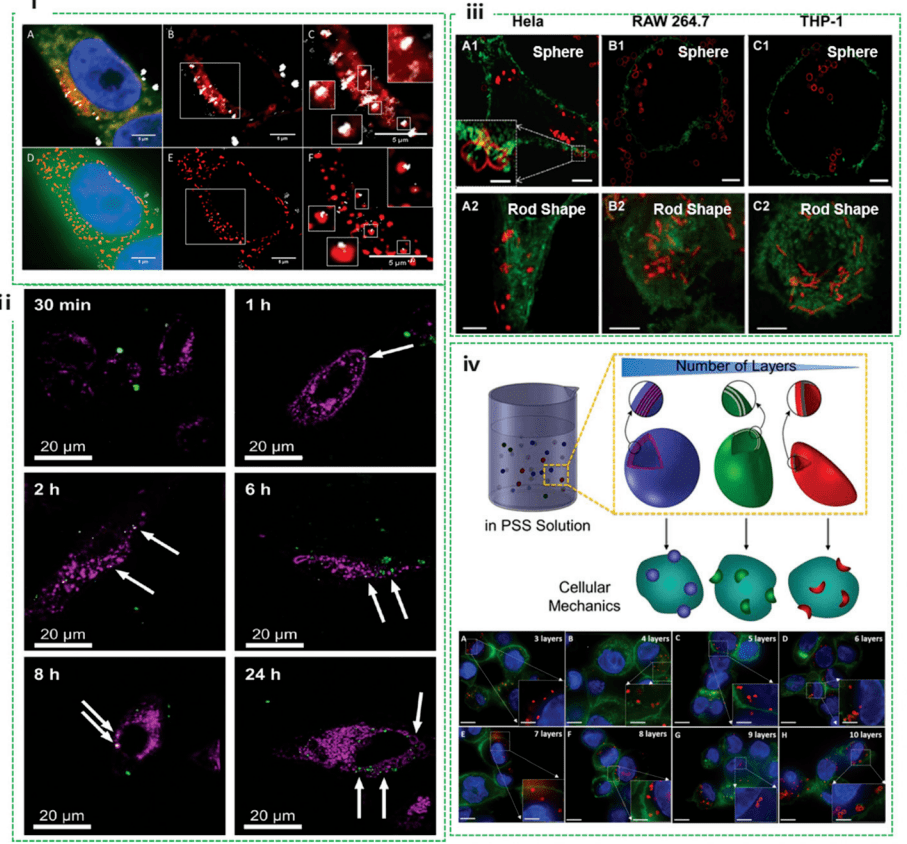
Figure 2. Nanocellular interactions studied by SIM, including colocalization of nanoparticles, intracellular deformation of nanocapsules, and quantification of internalized nanocapsules
By using a SIM, the researchers captured the process of metal-organic frameworks (MOFs) entering HeLa cells within 24 hours. The results showed that MOF in the extracellular space moves faster than the MOF located within the cell due to mediator differences between intracellular and extracellular spaces (Figure 2ii). Further studies found that the uptake of MOFs into HeLa cells was not affected either by drug model (calcein) loading or by temperature processing. The deformation properties of nanoparticles during internalization provide insight into their stability and potential mechanisms of action within cells. However, using conventional confocal microscopy, it is often challenging to identify subtle changes in nanostructure morphology when interacting with biological systems. Scholars explored SIM to study intracellular deformation of polymer capsules in various cell lines. It has been observed that the deformation of the capsule depends on the cell line (Figure 2iii), including HeLa, Raw264.7, and different THP-1s, but is independent of the shape of the capsule (spheroid and rod-shaped). This suggests that mechanical forces vary from cell line to cell line, as the highest percentage of deformation occurs in HeLa, followed by RAW264.7 and differentiated THP-1 cells. In addition, they precisely quantified the cellular internalization mechanism that distinguishes THP-1 cells with polymer nanocapsules. The pressure exerted on the capsule during cell internalization is quantified by assessing the degree of capsule deformation visualized by SIM (Figure 2iv). This pioneering work provides a practical approach to examining cellular mechanics, which has important implications for revealing cellular mechanobiology and designing advanced materials that are sensitive to cellular mechanical forces.
2.2 Stimulated emission loss microscopy (STED)
The fluorophores in the outer region of the STED depletion point diffusion function sharpen the focal point, thus increasing the transverse and axial resolution to 25-80 nm (Figure 1). With SIM, STED allows for higher resolution, no post-math processing, and the ability to image in real time and acquire quickly. For example, STED microscopy has been successfully used to image the localization of carbon dots in fixed and living MCF7 cells, with more than 6-fold spatial resolution improvements down to 30 nm compared to traditional confocal microscopy (Figure 3i). Similarly, STED is also used to image the interaction of living cells with transferrin. Compared to confocal images, nanoparticles have approximately 4x resolution enhancement. This STED not only enables precise imaging of these protein-based structures in living cells, but also reveals the enrichment of particles after internalization of early endosomal structures (Figure 3ii). Kraegeloh's group first applied STED to study nanoparticle-cell interactions. They used STED to determine the aggregate size of fluorescent silica particles (32 and 83 nm in diameter) within human colon cancer cells (Caco-2). Quantitative assessment of 32 nm particle migration compared to 83 nm particles showed that more 32 nm particles were internalized and they migrated faster into cells. Only 32 nm granules penetrate the nucleus and form 200 nm agglomerates after 48 h and 300 nm agglomerates in the nucleus after 72 h (Figure 3iii). This work opens up a pathway to study the pleiotropic interactions of nanomaterials. Kraegeloh's group is the first to further demonstrate that quantitative estimation of the number of silica nanoparticles internalized by lung epithelial cells (A549) can be extracted from the entire cell's 3D STED image stack by image processing. STED images were taken 5 h after A549 cells were ingested into nanoparticles with a diameter of 25 nm (Figure 3iv).
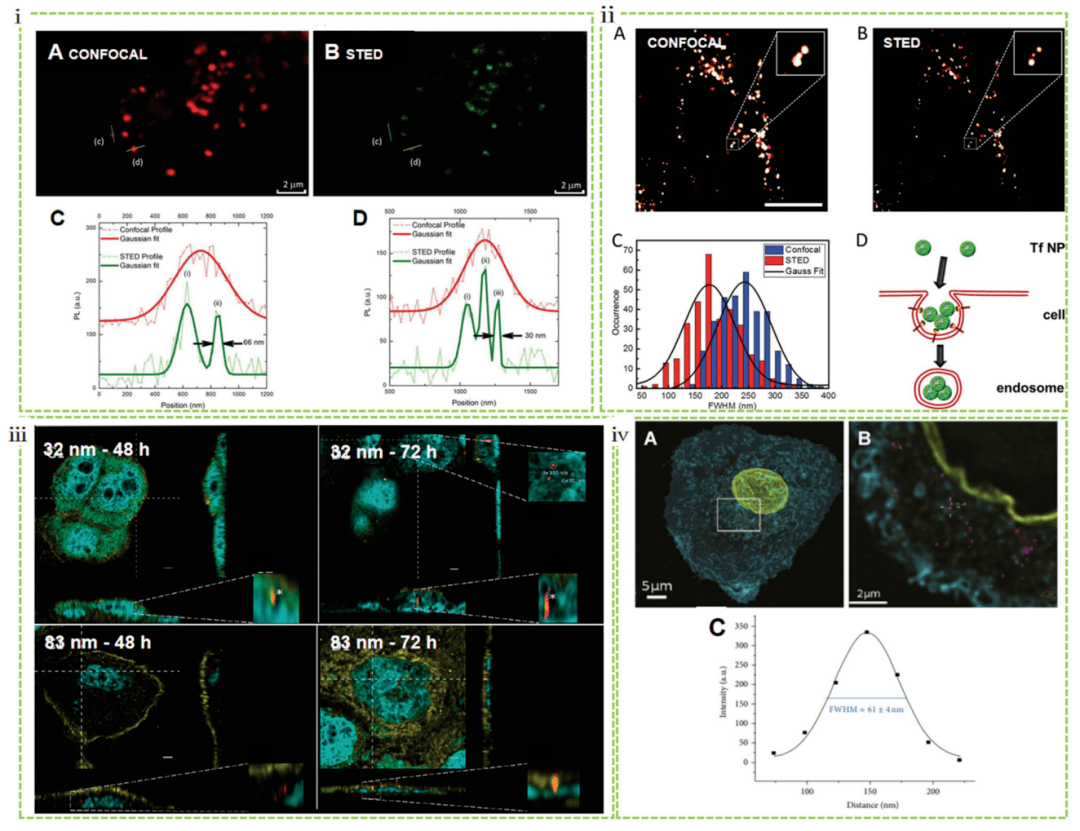
Figure 3 Nanocellular interactions studied by STED, including endocytosis mechanisms and internalization quantification of nanoparticles
2.3 Stochastic optical reconstruction microscope (STORM)
Based on the accurate localization of a single random scintillation fluorophore, STORM provides high-resolution images with tens of times higher resolution than standard fluorescence microscopy. STORM has been successfully used entirely in biological research to discover unrevealed cellular structures. De Geest and Albertazzi combined STORM with single-molecule data analysis to explore the behavior of nanoparticles as they enter cells, providing quantitative studies of nanoparticle interactions related to cell membranes and subsequent uptake mechanisms. Notably, they directly compared images of intracellular nanoparticles obtained using TEM, STORM, and confocal microscopy (Figure 4i). STORM has also recently been used in quantitative in vitro studies of nanobiological interactions, in which researchers have investigated how the formation of a protein crown surrounding nanoparticles affects the stability, targeting ability, and immunogenicity of injected nanoparticles to realize their therapeutic potential. Albertazzi's group demonstrated that subtle initial differences in surface chemistry between MSNs can lead to a diversity of heterogeneous protein amplification particles, leading to the coexistence of particles with different protein layers and different physicochemical properties (Figure 4ii).
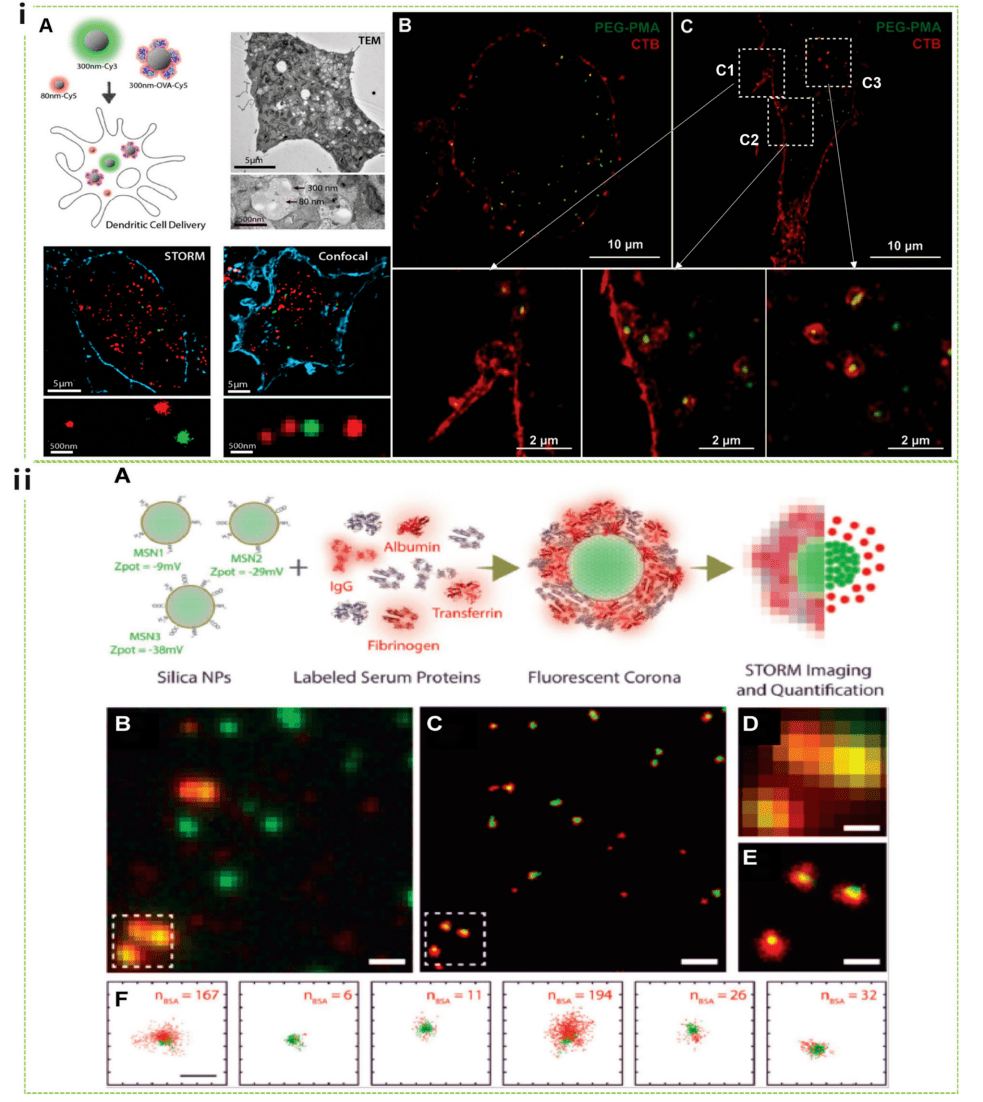
Figure 4 Nanocellular interactions studied by STORM, including uptake mechanisms and protein crown formation
2.4 Photoactivated localization microscopy (PALM)
PALM utilizes photoswitched fluorescent proteins to activate molecular subsets for random-ordered single-molecule recognition. A promising application of live-cell PALM is the use of photoactivation to perform high-density single-particle tracking, overcoming the traditional limitations of single-particle tracking to work with systems that display very low concentrations of fluorophores. Dynamic endocytosis of polystyrene-based nanoparticle endocytosis was investigated by using PALM combined with single-particle tracing, which allows visualization of clathrin-mediated nanoparticle endocytosis in a living cell environment. The results show that in most events, > 90% of the nanoparticles first bind to the cell membrane and subsequently form cypermethrin-coated pits (CCPs), while the remaining nanoparticles diffuse within the cell membrane for utilization by prefabricated CCPs.
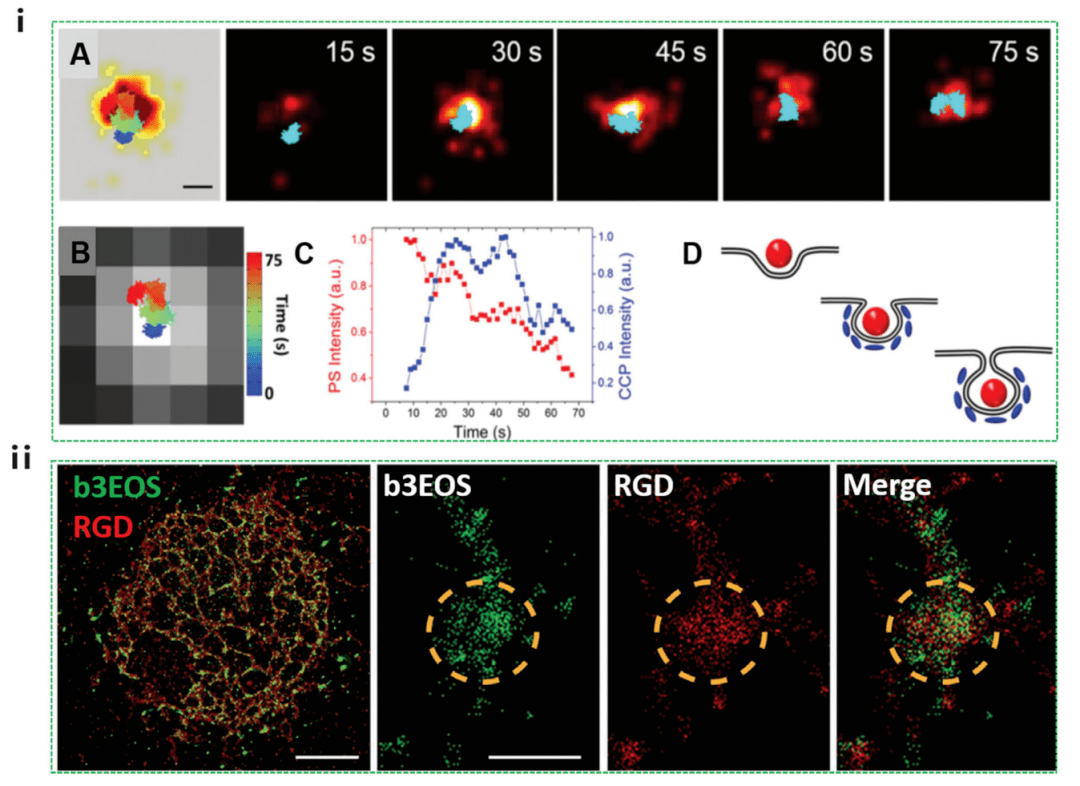
Figure 5 Nanocellular interactions studied by PALM, including cellular uptake processes and intracellular localization
As shown in Figure 5i, the nanoparticles (red) first adhere to the plasma membrane, while clathrin (blue) is not visible. The CCP signal then gradually strengthens, indicating that CLA-THRIN is recruiting to the site. During this duration of 55 ± 23 seconds, the emission intensity of the nanoparticles remained largely unchanged. After the CCP signal peaks, the nanoparticle intensity begins to decrease. In the later stages (45-75 sec), lateral movements of 100 nm were observed in both the PALM image of the CCP and the nanoparticle trajectories, possibly due to the nanoparticles in the intracellular region. While this work focused only on clathrin-mediated endocytosis, it opens up an avenue for studying other endocytosis mechanisms of action of PALM nanoparticles. PALM has also been used to characterize and quantify early integrinin clusters on RGD matrices of varying stiffness at 10 nm resolution (Figure 5ii). The results showed that very early adhesions consisted of 50 100 nm clusters of β3-activated integrin proteins. These early adhesions form similarly on flexible and rigid substrates, but most adhesion to rigid substrates is temporary.
02 Research Summary
Over the past decade, the field of light microscopy in nanocellular interactions has experienced a renaissance with the integration of super-resolution techniques. Although there are still some concerns that super-resolution microscopy, like other fluorescence microscopy techniques, relies on the expression of fluorescent molecules within cells, often through the introduction of external genes that directly affect cell physiology, and therefore may not reflect "real" nanocellular interactions, there is no doubt that the development of super-resolution technology represents a major advance and a powerful tool for understanding the behavior of nanomaterials in the biological field.
The ability to directly visualize nanomaterials at nanoscale resolution yields critical information such as the mechanism of individual endocytic events, the tracking of intracellular nanoparticles, and the intracellular location of therapeutic payloads. These mechanisms of action must be understood in order to design safe nanoparticles with enhanced internalization efficiency and tracking kinetics. In turn, such information can be used to deepen a basic understanding of the biological environment. As super-resolution microscopy techniques such as better detectors and fluorescent probes continue to evolve, we have seen significant improvements in the performance of this method in terms of spatial and temporal resolution, accessibility of complex specimens, and minimally invasive of living systems. This will provide more opportunities to study nanocellular interactions.
In this study, the discovery of the 2014 Nobel Prize in Chemistry has been industrialized in China. Ningbo Lixian Intelligent Technology Co., Ltd. (INVIEW) has released the ultra-high-resolution microscopy system iSTORM, which adopts 3D random optical reconstruction technology, high-precision cell real-time locking technology, multi-channel simultaneous imaging technology, etc., and has been highly recognized by more than 50 scientific research groups and more than 100 researchers for its excellent characteristics such as nanoscale observation accuracy, high stability, wide environmental application, fast imaging, and easy operation.
References:
1. Advances in super-resolution fluorescence microscopy for the study of nano–cell interactions
About Us:
Ningbo Lixian Intelligent Technology Co., Ltd. (INVIEW) is a scientific and technological enterprise specializing in ultra-high resolution microscopy technology and product research and development, relying on the automatic control, new generation information technology of Fudan University and the biology, optics, image processing and other technologies of the Hong Kong University of Science and Technology, with optical, biological, automatic control, mechanical, information technology and other fields of interdisciplinary technical team, the 2014 Nobel Prize in Chemistry technology industrialization, launched ultra-high resolution microscopy products. Help people see the microscopic world from an unprecedented perspective, push the limits, and see things like never before.


